Geography 140
Introduction to Physical Geography
Lecture: Vertical Thermal Structure of the Atmosphere

IV. The atmosphere has a vertical thermal structure as well as a vertical
pressure structure.
A. The atmosphere has been divided into layers according to the behavior
of temperatures in their relationship to altitude. In this lecture,
I'll start from the ground and move up through the atmosphere.
![[ vertical temperature structure of the atmosphere ]](tprofile.jpg) B. The lowest layer is the troposphere, the layer in which we live and in
which our weather is experienced. In fact, "troposphere" means the
realm of mixing, because air is vigorously mixed and stirred here by
storms, convection, and wind systems.
1. It extends up roughly 10 km (oh, about 6 miles-ish).
2. It is characterized by an inverse relationship between air
temperatures and altitude: Temperatures drop as you climb up in
the troposphere.
a. In still air, it cools by an average of 6.5° C for every
kilometer (1,000 meters) gain in elevation. So, if it's a
gorgeous, sunny, toasty day at Beach, 30° C (~86° F),
it'll be about 23.5° C (room temperature) on top of the
Santa Monicas out near the Ventura County line.
b. This drop in temperatures with a rise in elevation is called the
"normal lapse rate." It represents an average, not the actual
lapse rate at a particular place and time.
3. The tropopause is the top of the troposphere: The troposphere stops
here.
a. It is situated about 10 km up.
i. It's more like 8 km (5 mi.) over the polar regions in
winter (cold air tends to settle downward) and 18 km (11
mi.) over the equatorial regions, due to greater convection
there (heating caused by the direct rays of the sun).
ii. In the mid-latitudes, it's lower in winter and higher in
summer, for the same sorts of reasons.
B. The lowest layer is the troposphere, the layer in which we live and in
which our weather is experienced. In fact, "troposphere" means the
realm of mixing, because air is vigorously mixed and stirred here by
storms, convection, and wind systems.
1. It extends up roughly 10 km (oh, about 6 miles-ish).
2. It is characterized by an inverse relationship between air
temperatures and altitude: Temperatures drop as you climb up in
the troposphere.
a. In still air, it cools by an average of 6.5° C for every
kilometer (1,000 meters) gain in elevation. So, if it's a
gorgeous, sunny, toasty day at Beach, 30° C (~86° F),
it'll be about 23.5° C (room temperature) on top of the
Santa Monicas out near the Ventura County line.
b. This drop in temperatures with a rise in elevation is called the
"normal lapse rate." It represents an average, not the actual
lapse rate at a particular place and time.
3. The tropopause is the top of the troposphere: The troposphere stops
here.
a. It is situated about 10 km up.
i. It's more like 8 km (5 mi.) over the polar regions in
winter (cold air tends to settle downward) and 18 km (11
mi.) over the equatorial regions, due to greater convection
there (heating caused by the direct rays of the sun).
ii. In the mid-latitudes, it's lower in winter and higher in
summer, for the same sorts of reasons.
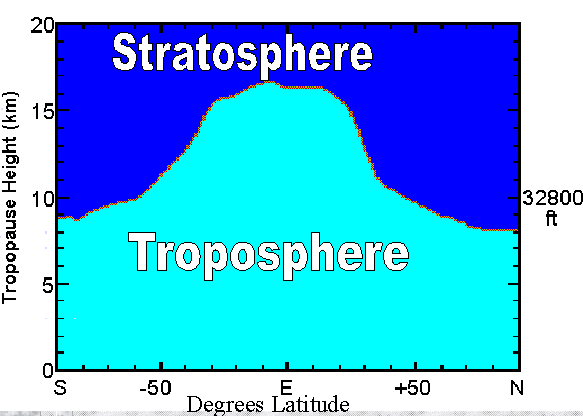 b. At the tropopause, temperatures stop dropping with gains in
altitude.
i. The normal lapse rate no longer applies.
ii. In fact, temperatures do nothing as altitude increases:
The tropopause could be described as an "isothermal" belt
(a zone having the same temperatures as you move up).
iii. Temperatures at the tropopause are pretty cold: about
-50° C (roughly -120° F).
c. The tropopause, then, separates the intensely mixed air of the
troposphere from the much quieter air of the stratosphere (home
of the ozone layer, which you met before).
C. The stratosphere is the next major division.
1. It extends from the tropopause up to about 50 km.
2. It is characterized by a direct relationship between temperatures
and altitude: Temperatures climb as you climb.
a. The top of the stratosphere is called, yes, you guessed it, the
stratopause, another isothermal belt.
b. By the time you get to the stratopause, temperatures have warmed
up to freezing or close to it!
c. This warming with altitude has to do with ... ... the presence
of the ozone layer in the stratosphere. Remember the ozone
layer, up there from, oh, 20 to 50 km? Ozone absorbs high
energy, shortwave, ultraviolet radiation from the sun.
Absorption of energy heats the absorbing object, and so it is
here: Ozone heats up by absorbing UV radiation, and that
accounts for the climb in temperatures with a climb in altitude
here in the stratosphere.
D. The mesosphere is the layer above the stratopause.
1. It extends up from the stratopause to about 80 km.
2. It is characterized by resumption of an inverse relationship
between temperature and altitude: Temperatures go back to dropping
as you climb.
3. And do they ever drop! They get down to nearly -100° C
(~200° F). This is the coldest layer in the atmosphere.
4. That low temperature is attained at the mesopause, which tops the
mesosphere.
5. In a manner of thinking, the mesosphere is a resumption of the
troposphere after the rude interruption of the ozone layer in the
stratosphere.
6. The difference is that the mesosphere is not in contact with the
forces mixing up the air in the troposphere, so it's much quieter
up there.
E. The thermosphere is the last thermally defined layer of the
atmosphere.
1. It is characterized by a direct relationship between temperature
and altitude.
2. Temperatures get up to 725° - 1,225° C.
3. This sounds terribly impressive, but you have to remember that
there are so few molecules and ions up there to get excited to
motion levels corresponding to 725° - 1,225° C. If you
were up there, it would feel pretty cold as you got yourself a
nasty dose of radiation, because there are so very few molecules to
transfer energy to your skin. If you were up there, bare-skinned,
you'd have bigger things to worry about than the measured air
temperature!
4. The thermosphere can be further subdivided on the basis of physico-
chemistry, kind of like when we noted that the (thermally-defined)
stratosphere contains the (chemically-defined) ozone layer.
a. The lower thermosphere is called the ionosphere.
i. The ionosphere extends from roughly 80 km (50 mi.) to
somewhere around 300 to 600 km out (~185 - 375 mi.).
ii. It is the first line of defense for Earth against extremely
short wave radiation (e.g., UV-B and UV-C) and, to a lesser
extent, high energy particles from the sun and cosmic
"rays." These particles are ionized atoms, that is, atoms
with missing electrons, including isolated protons and
alpha-particles (two protons with two neutrons and no
electrons). Cosmic "rays" are deadly to life on Earth.
iii. These rays and really high energy, fast-moving particles
smash into the few molecules of the ionosphere with such
force that they strip them of electrons, turning them into
ions, or electrically-imbalanced atoms, too.
iv. The ions, with their electrical imbalances, are drawn by
the earth's magnetic field and align themselves with that
field's lines of force, rather the way iron filings align
themselves with the magnetic field lines on a sheet of
paper lying above a magnet. This means that they are
really concentrated in great abundance where those magnetic
field lines converge, at the north and south magnetic
poles.
iv. One side effect of this process and of further interactions
between Earth's ions and those from the sun or outer space
is a cascade of lower energy particles, including visible
light photons. This produces the aurorae, which are often
visible in high latitude regions. They look like glows,
rays, arcs, and even curtain-like veils (which even move,
kind of the way a curtain will move in an open window).
They're usually greenish, but other colors are sometimes
seen.
b. At the tropopause, temperatures stop dropping with gains in
altitude.
i. The normal lapse rate no longer applies.
ii. In fact, temperatures do nothing as altitude increases:
The tropopause could be described as an "isothermal" belt
(a zone having the same temperatures as you move up).
iii. Temperatures at the tropopause are pretty cold: about
-50° C (roughly -120° F).
c. The tropopause, then, separates the intensely mixed air of the
troposphere from the much quieter air of the stratosphere (home
of the ozone layer, which you met before).
C. The stratosphere is the next major division.
1. It extends from the tropopause up to about 50 km.
2. It is characterized by a direct relationship between temperatures
and altitude: Temperatures climb as you climb.
a. The top of the stratosphere is called, yes, you guessed it, the
stratopause, another isothermal belt.
b. By the time you get to the stratopause, temperatures have warmed
up to freezing or close to it!
c. This warming with altitude has to do with ... ... the presence
of the ozone layer in the stratosphere. Remember the ozone
layer, up there from, oh, 20 to 50 km? Ozone absorbs high
energy, shortwave, ultraviolet radiation from the sun.
Absorption of energy heats the absorbing object, and so it is
here: Ozone heats up by absorbing UV radiation, and that
accounts for the climb in temperatures with a climb in altitude
here in the stratosphere.
D. The mesosphere is the layer above the stratopause.
1. It extends up from the stratopause to about 80 km.
2. It is characterized by resumption of an inverse relationship
between temperature and altitude: Temperatures go back to dropping
as you climb.
3. And do they ever drop! They get down to nearly -100° C
(~200° F). This is the coldest layer in the atmosphere.
4. That low temperature is attained at the mesopause, which tops the
mesosphere.
5. In a manner of thinking, the mesosphere is a resumption of the
troposphere after the rude interruption of the ozone layer in the
stratosphere.
6. The difference is that the mesosphere is not in contact with the
forces mixing up the air in the troposphere, so it's much quieter
up there.
E. The thermosphere is the last thermally defined layer of the
atmosphere.
1. It is characterized by a direct relationship between temperature
and altitude.
2. Temperatures get up to 725° - 1,225° C.
3. This sounds terribly impressive, but you have to remember that
there are so few molecules and ions up there to get excited to
motion levels corresponding to 725° - 1,225° C. If you
were up there, it would feel pretty cold as you got yourself a
nasty dose of radiation, because there are so very few molecules to
transfer energy to your skin. If you were up there, bare-skinned,
you'd have bigger things to worry about than the measured air
temperature!
4. The thermosphere can be further subdivided on the basis of physico-
chemistry, kind of like when we noted that the (thermally-defined)
stratosphere contains the (chemically-defined) ozone layer.
a. The lower thermosphere is called the ionosphere.
i. The ionosphere extends from roughly 80 km (50 mi.) to
somewhere around 300 to 600 km out (~185 - 375 mi.).
ii. It is the first line of defense for Earth against extremely
short wave radiation (e.g., UV-B and UV-C) and, to a lesser
extent, high energy particles from the sun and cosmic
"rays." These particles are ionized atoms, that is, atoms
with missing electrons, including isolated protons and
alpha-particles (two protons with two neutrons and no
electrons). Cosmic "rays" are deadly to life on Earth.
iii. These rays and really high energy, fast-moving particles
smash into the few molecules of the ionosphere with such
force that they strip them of electrons, turning them into
ions, or electrically-imbalanced atoms, too.
iv. The ions, with their electrical imbalances, are drawn by
the earth's magnetic field and align themselves with that
field's lines of force, rather the way iron filings align
themselves with the magnetic field lines on a sheet of
paper lying above a magnet. This means that they are
really concentrated in great abundance where those magnetic
field lines converge, at the north and south magnetic
poles.
iv. One side effect of this process and of further interactions
between Earth's ions and those from the sun or outer space
is a cascade of lower energy particles, including visible
light photons. This produces the aurorae, which are often
visible in high latitude regions. They look like glows,
rays, arcs, and even curtain-like veils (which even move,
kind of the way a curtain will move in an open window).
They're usually greenish, but other colors are sometimes
seen.
 Dick Hutchinson of Circle, Alaska, took this picture, and
you can see more of his work with aurorae there at:
http://www.ptialaska.net/~hutch/aurora.html.
a. These light displays are called the aurora borealis or
Northern Lights in the Northern Hemisphere and the
aurora australis or Southern Lights in the Southern
Hemisphere.
b. Normally, you have to be near the polar regions to see
them but, every once in a while, especially during the
peaks of the sunspot cycle (when the sun is emitting a
stronger solar wind of charged particles), they have
been seen at lower latitudes, including even in the
tropics! Even if they were apparent at our latitude of
34° N, we would probably miss the show, though,
because of light pollution from our city lights: You
would have to be out in the mountains or desert to see
them if they were down in our low latitudes.
v. Another side effect is that the ionosphere absorbs and
reflects radio waves. A layer of ions at the very bottom
of the ionosphere (largely oxygen) forms in the daytime and
can absorb a lot of radio waves. It disappears at night,
but dense ion layers higher up last longer into the night
and reflect radio waves, espeially short wave radio. This
allows radio stations to be picked up way beyond the
horizon from the broadcast tower, which is why you can pick
up radio broadcasts from Nevada, Nebraska, and Iowa at
night sometimes.
b. The exosphere is the second, outer layer of the thermosphere
Dick Hutchinson of Circle, Alaska, took this picture, and
you can see more of his work with aurorae there at:
http://www.ptialaska.net/~hutch/aurora.html.
a. These light displays are called the aurora borealis or
Northern Lights in the Northern Hemisphere and the
aurora australis or Southern Lights in the Southern
Hemisphere.
b. Normally, you have to be near the polar regions to see
them but, every once in a while, especially during the
peaks of the sunspot cycle (when the sun is emitting a
stronger solar wind of charged particles), they have
been seen at lower latitudes, including even in the
tropics! Even if they were apparent at our latitude of
34° N, we would probably miss the show, though,
because of light pollution from our city lights: You
would have to be out in the mountains or desert to see
them if they were down in our low latitudes.
v. Another side effect is that the ionosphere absorbs and
reflects radio waves. A layer of ions at the very bottom
of the ionosphere (largely oxygen) forms in the daytime and
can absorb a lot of radio waves. It disappears at night,
but dense ion layers higher up last longer into the night
and reflect radio waves, espeially short wave radio. This
allows radio stations to be picked up way beyond the
horizon from the broadcast tower, which is why you can pick
up radio broadcasts from Nevada, Nebraska, and Iowa at
night sometimes.
b. The exosphere is the second, outer layer of the thermosphere
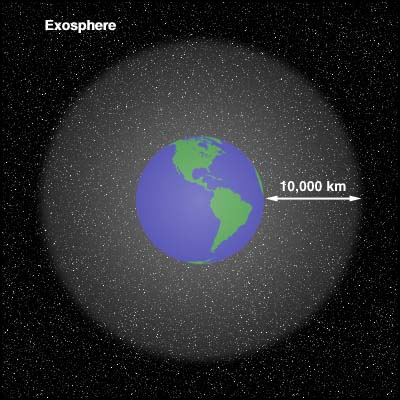 i. The exosphere lies beyond about 500-1,000 km and is
characterized by increasing hydrogen and helium content,
because the oxygen and nitrogen that dominate the lower
atmosphere have been dissociated into ions in the
ionosphere. We're not talking too many molecules and ions
way the heck up here. Density shades into the levels seen
in interplanetary space up around, oh, 10,000 km.
ii. Hydrogen and helium molecules can and do easily leave Earth
orbit for outer space from the top of the exosphere,
because at this altitude, when their trajectories are
changed by collision with another molecule to a generally
upward direction, they have less and less chance of
colliding with another one and being bounced back
earthward. So, there is a constant loss of H and He to
outer space from the upper exosphere, and Earth's gravity
is not strong enough to hold onto these lightest of all
gasses (unlike such big bruisers as Jupiter, Saturn,
Uranus, and Neptune, the gas giant planets in the outer
solar system, which retain hydrogen and helium atmospheres
with their tremendous size and gravity).
V. So, you've seen that the atmosphere has a distinct vertical structure,
which can be described in terms of a pressure gradient, as in the last
lecture, and in terms of its thermal structure and its physico-chemical
structure. I organized this particular lecture around the thermal
structure of the atmosphere but included discussion of the physico-
chemical layers within the thermal layers where they're found. So, the
ozone layer was discussed as part of the stratosphere, while the
ionosphere and exosphere were discussed under the thermosphere. There is
another distinction often made, too: We can differentiate the vertical
structure of the atmosphere by the balance of chemical mixing and
ionization:
A. The homosphere is that portion of the lower atmosphere with almost no
ionization and in which there are mixing mechanisms moving gases from
one part of the atmosphere to another, resulting in a fairly uniform
chemical composition: This is the lower 80-100 km or thereabouts
(troposphere through mesosphere).
1. The major variation you get in here is a greater concentration of
ozone in the stratosphere, but ozone is a non-ionized gas, even so.
2. Throughout the homosphere, then, nitrogen and oxygen gasses
dominate.
B. The heterosphere is the area in which you get varying mixes of gas
molecules and ions, and in which the nitrogen and oxygen that dominate
the lower atmosphere give way, through ionization, to hydrogen and
helium at the top of the atmosphere. That would be somewhere from 80
km to 10,000 km, where the hydrogen and helium gasses are so feebly
held to Earth that they are constantly being Lost in Space.
Come away from this lecture knowing the four different thermally-defined
layers of the atmosphere (troposphere, statosphere, mesosphere, and
thermosphere) and what sort of relation (direct or inverse) between
temperature and altitude is found in each of them. Know the three isothermic
belts (tropopause, stratopause, and mesopause) and what the dickens an
"isothermic belt" is.
Learn which physico-chemical feature is found in the four layers (weather in
the troposphere, ozone layer in the stratosphere, and the ionosphere and
exosphere in the thermosphere), and be able to explain the aurorae (Northern
Lights or aurora borealis and Southern Lights or aurora australis). Also,
know the difference between the homosphere and the heterosphere and which
thermally-defined layers belong to which.
i. The exosphere lies beyond about 500-1,000 km and is
characterized by increasing hydrogen and helium content,
because the oxygen and nitrogen that dominate the lower
atmosphere have been dissociated into ions in the
ionosphere. We're not talking too many molecules and ions
way the heck up here. Density shades into the levels seen
in interplanetary space up around, oh, 10,000 km.
ii. Hydrogen and helium molecules can and do easily leave Earth
orbit for outer space from the top of the exosphere,
because at this altitude, when their trajectories are
changed by collision with another molecule to a generally
upward direction, they have less and less chance of
colliding with another one and being bounced back
earthward. So, there is a constant loss of H and He to
outer space from the upper exosphere, and Earth's gravity
is not strong enough to hold onto these lightest of all
gasses (unlike such big bruisers as Jupiter, Saturn,
Uranus, and Neptune, the gas giant planets in the outer
solar system, which retain hydrogen and helium atmospheres
with their tremendous size and gravity).
V. So, you've seen that the atmosphere has a distinct vertical structure,
which can be described in terms of a pressure gradient, as in the last
lecture, and in terms of its thermal structure and its physico-chemical
structure. I organized this particular lecture around the thermal
structure of the atmosphere but included discussion of the physico-
chemical layers within the thermal layers where they're found. So, the
ozone layer was discussed as part of the stratosphere, while the
ionosphere and exosphere were discussed under the thermosphere. There is
another distinction often made, too: We can differentiate the vertical
structure of the atmosphere by the balance of chemical mixing and
ionization:
A. The homosphere is that portion of the lower atmosphere with almost no
ionization and in which there are mixing mechanisms moving gases from
one part of the atmosphere to another, resulting in a fairly uniform
chemical composition: This is the lower 80-100 km or thereabouts
(troposphere through mesosphere).
1. The major variation you get in here is a greater concentration of
ozone in the stratosphere, but ozone is a non-ionized gas, even so.
2. Throughout the homosphere, then, nitrogen and oxygen gasses
dominate.
B. The heterosphere is the area in which you get varying mixes of gas
molecules and ions, and in which the nitrogen and oxygen that dominate
the lower atmosphere give way, through ionization, to hydrogen and
helium at the top of the atmosphere. That would be somewhere from 80
km to 10,000 km, where the hydrogen and helium gasses are so feebly
held to Earth that they are constantly being Lost in Space.
Come away from this lecture knowing the four different thermally-defined
layers of the atmosphere (troposphere, statosphere, mesosphere, and
thermosphere) and what sort of relation (direct or inverse) between
temperature and altitude is found in each of them. Know the three isothermic
belts (tropopause, stratopause, and mesopause) and what the dickens an
"isothermic belt" is.
Learn which physico-chemical feature is found in the four layers (weather in
the troposphere, ozone layer in the stratosphere, and the ionosphere and
exosphere in the thermosphere), and be able to explain the aurorae (Northern
Lights or aurora borealis and Southern Lights or aurora australis). Also,
know the difference between the homosphere and the heterosphere and which
thermally-defined layers belong to which.
http://www.csulb.edu/~rodrigue/geog140/lectures/thermalstructure.html


![[ vertical temperature structure of the atmosphere ]](tprofile.jpg) B. The lowest layer is the troposphere, the layer in which we live and in
which our weather is experienced. In fact, "troposphere" means the
realm of mixing, because air is vigorously mixed and stirred here by
storms, convection, and wind systems.
1. It extends up roughly 10 km (oh, about 6 miles-ish).
2. It is characterized by an inverse relationship between air
temperatures and altitude: Temperatures drop as you climb up in
the troposphere.
a. In still air, it cools by an average of 6.5° C for every
kilometer (1,000 meters) gain in elevation. So, if it's a
gorgeous, sunny, toasty day at Beach, 30° C (~86° F),
it'll be about 23.5° C (room temperature) on top of the
Santa Monicas out near the Ventura County line.
b. This drop in temperatures with a rise in elevation is called the
"normal lapse rate." It represents an average, not the actual
lapse rate at a particular place and time.
3. The tropopause is the top of the troposphere: The troposphere stops
here.
a. It is situated about 10 km up.
i. It's more like 8 km (5 mi.) over the polar regions in
winter (cold air tends to settle downward) and 18 km (11
mi.) over the equatorial regions, due to greater convection
there (heating caused by the direct rays of the sun).
ii. In the mid-latitudes, it's lower in winter and higher in
summer, for the same sorts of reasons.
B. The lowest layer is the troposphere, the layer in which we live and in
which our weather is experienced. In fact, "troposphere" means the
realm of mixing, because air is vigorously mixed and stirred here by
storms, convection, and wind systems.
1. It extends up roughly 10 km (oh, about 6 miles-ish).
2. It is characterized by an inverse relationship between air
temperatures and altitude: Temperatures drop as you climb up in
the troposphere.
a. In still air, it cools by an average of 6.5° C for every
kilometer (1,000 meters) gain in elevation. So, if it's a
gorgeous, sunny, toasty day at Beach, 30° C (~86° F),
it'll be about 23.5° C (room temperature) on top of the
Santa Monicas out near the Ventura County line.
b. This drop in temperatures with a rise in elevation is called the
"normal lapse rate." It represents an average, not the actual
lapse rate at a particular place and time.
3. The tropopause is the top of the troposphere: The troposphere stops
here.
a. It is situated about 10 km up.
i. It's more like 8 km (5 mi.) over the polar regions in
winter (cold air tends to settle downward) and 18 km (11
mi.) over the equatorial regions, due to greater convection
there (heating caused by the direct rays of the sun).
ii. In the mid-latitudes, it's lower in winter and higher in
summer, for the same sorts of reasons.
 b. At the tropopause, temperatures stop dropping with gains in
altitude.
i. The normal lapse rate no longer applies.
ii. In fact, temperatures do nothing as altitude increases:
The tropopause could be described as an "isothermal" belt
(a zone having the same temperatures as you move up).
iii. Temperatures at the tropopause are pretty cold: about
-50° C (roughly -120° F).
c. The tropopause, then, separates the intensely mixed air of the
troposphere from the much quieter air of the stratosphere (home
of the ozone layer, which you met before).
C. The stratosphere is the next major division.
1. It extends from the tropopause up to about 50 km.
2. It is characterized by a direct relationship between temperatures
and altitude: Temperatures climb as you climb.
a. The top of the stratosphere is called, yes, you guessed it, the
stratopause, another isothermal belt.
b. By the time you get to the stratopause, temperatures have warmed
up to freezing or close to it!
c. This warming with altitude has to do with ... ... the presence
of the ozone layer in the stratosphere. Remember the ozone
layer, up there from, oh, 20 to 50 km? Ozone absorbs high
energy, shortwave, ultraviolet radiation from the sun.
Absorption of energy heats the absorbing object, and so it is
here: Ozone heats up by absorbing UV radiation, and that
accounts for the climb in temperatures with a climb in altitude
here in the stratosphere.
D. The mesosphere is the layer above the stratopause.
1. It extends up from the stratopause to about 80 km.
2. It is characterized by resumption of an inverse relationship
between temperature and altitude: Temperatures go back to dropping
as you climb.
3. And do they ever drop! They get down to nearly -100° C
(~200° F). This is the coldest layer in the atmosphere.
4. That low temperature is attained at the mesopause, which tops the
mesosphere.
5. In a manner of thinking, the mesosphere is a resumption of the
troposphere after the rude interruption of the ozone layer in the
stratosphere.
6. The difference is that the mesosphere is not in contact with the
forces mixing up the air in the troposphere, so it's much quieter
up there.
E. The thermosphere is the last thermally defined layer of the
atmosphere.
1. It is characterized by a direct relationship between temperature
and altitude.
2. Temperatures get up to 725° - 1,225° C.
3. This sounds terribly impressive, but you have to remember that
there are so few molecules and ions up there to get excited to
motion levels corresponding to 725° - 1,225° C. If you
were up there, it would feel pretty cold as you got yourself a
nasty dose of radiation, because there are so very few molecules to
transfer energy to your skin. If you were up there, bare-skinned,
you'd have bigger things to worry about than the measured air
temperature!
4. The thermosphere can be further subdivided on the basis of physico-
chemistry, kind of like when we noted that the (thermally-defined)
stratosphere contains the (chemically-defined) ozone layer.
a. The lower thermosphere is called the ionosphere.
i. The ionosphere extends from roughly 80 km (50 mi.) to
somewhere around 300 to 600 km out (~185 - 375 mi.).
ii. It is the first line of defense for Earth against extremely
short wave radiation (e.g., UV-B and UV-C) and, to a lesser
extent, high energy particles from the sun and cosmic
"rays." These particles are ionized atoms, that is, atoms
with missing electrons, including isolated protons and
alpha-particles (two protons with two neutrons and no
electrons). Cosmic "rays" are deadly to life on Earth.
iii. These rays and really high energy, fast-moving particles
smash into the few molecules of the ionosphere with such
force that they strip them of electrons, turning them into
ions, or electrically-imbalanced atoms, too.
iv. The ions, with their electrical imbalances, are drawn by
the earth's magnetic field and align themselves with that
field's lines of force, rather the way iron filings align
themselves with the magnetic field lines on a sheet of
paper lying above a magnet. This means that they are
really concentrated in great abundance where those magnetic
field lines converge, at the north and south magnetic
poles.
iv. One side effect of this process and of further interactions
between Earth's ions and those from the sun or outer space
is a cascade of lower energy particles, including visible
light photons. This produces the aurorae, which are often
visible in high latitude regions. They look like glows,
rays, arcs, and even curtain-like veils (which even move,
kind of the way a curtain will move in an open window).
They're usually greenish, but other colors are sometimes
seen.
b. At the tropopause, temperatures stop dropping with gains in
altitude.
i. The normal lapse rate no longer applies.
ii. In fact, temperatures do nothing as altitude increases:
The tropopause could be described as an "isothermal" belt
(a zone having the same temperatures as you move up).
iii. Temperatures at the tropopause are pretty cold: about
-50° C (roughly -120° F).
c. The tropopause, then, separates the intensely mixed air of the
troposphere from the much quieter air of the stratosphere (home
of the ozone layer, which you met before).
C. The stratosphere is the next major division.
1. It extends from the tropopause up to about 50 km.
2. It is characterized by a direct relationship between temperatures
and altitude: Temperatures climb as you climb.
a. The top of the stratosphere is called, yes, you guessed it, the
stratopause, another isothermal belt.
b. By the time you get to the stratopause, temperatures have warmed
up to freezing or close to it!
c. This warming with altitude has to do with ... ... the presence
of the ozone layer in the stratosphere. Remember the ozone
layer, up there from, oh, 20 to 50 km? Ozone absorbs high
energy, shortwave, ultraviolet radiation from the sun.
Absorption of energy heats the absorbing object, and so it is
here: Ozone heats up by absorbing UV radiation, and that
accounts for the climb in temperatures with a climb in altitude
here in the stratosphere.
D. The mesosphere is the layer above the stratopause.
1. It extends up from the stratopause to about 80 km.
2. It is characterized by resumption of an inverse relationship
between temperature and altitude: Temperatures go back to dropping
as you climb.
3. And do they ever drop! They get down to nearly -100° C
(~200° F). This is the coldest layer in the atmosphere.
4. That low temperature is attained at the mesopause, which tops the
mesosphere.
5. In a manner of thinking, the mesosphere is a resumption of the
troposphere after the rude interruption of the ozone layer in the
stratosphere.
6. The difference is that the mesosphere is not in contact with the
forces mixing up the air in the troposphere, so it's much quieter
up there.
E. The thermosphere is the last thermally defined layer of the
atmosphere.
1. It is characterized by a direct relationship between temperature
and altitude.
2. Temperatures get up to 725° - 1,225° C.
3. This sounds terribly impressive, but you have to remember that
there are so few molecules and ions up there to get excited to
motion levels corresponding to 725° - 1,225° C. If you
were up there, it would feel pretty cold as you got yourself a
nasty dose of radiation, because there are so very few molecules to
transfer energy to your skin. If you were up there, bare-skinned,
you'd have bigger things to worry about than the measured air
temperature!
4. The thermosphere can be further subdivided on the basis of physico-
chemistry, kind of like when we noted that the (thermally-defined)
stratosphere contains the (chemically-defined) ozone layer.
a. The lower thermosphere is called the ionosphere.
i. The ionosphere extends from roughly 80 km (50 mi.) to
somewhere around 300 to 600 km out (~185 - 375 mi.).
ii. It is the first line of defense for Earth against extremely
short wave radiation (e.g., UV-B and UV-C) and, to a lesser
extent, high energy particles from the sun and cosmic
"rays." These particles are ionized atoms, that is, atoms
with missing electrons, including isolated protons and
alpha-particles (two protons with two neutrons and no
electrons). Cosmic "rays" are deadly to life on Earth.
iii. These rays and really high energy, fast-moving particles
smash into the few molecules of the ionosphere with such
force that they strip them of electrons, turning them into
ions, or electrically-imbalanced atoms, too.
iv. The ions, with their electrical imbalances, are drawn by
the earth's magnetic field and align themselves with that
field's lines of force, rather the way iron filings align
themselves with the magnetic field lines on a sheet of
paper lying above a magnet. This means that they are
really concentrated in great abundance where those magnetic
field lines converge, at the north and south magnetic
poles.
iv. One side effect of this process and of further interactions
between Earth's ions and those from the sun or outer space
is a cascade of lower energy particles, including visible
light photons. This produces the aurorae, which are often
visible in high latitude regions. They look like glows,
rays, arcs, and even curtain-like veils (which even move,
kind of the way a curtain will move in an open window).
They're usually greenish, but other colors are sometimes
seen.
 Dick Hutchinson of Circle, Alaska, took this picture, and
you can see more of his work with aurorae there at:
http://www.ptialaska.net/~hutch/aurora.html.
a. These light displays are called the aurora borealis or
Northern Lights in the Northern Hemisphere and the
aurora australis or Southern Lights in the Southern
Hemisphere.
b. Normally, you have to be near the polar regions to see
them but, every once in a while, especially during the
peaks of the sunspot cycle (when the sun is emitting a
stronger solar wind of charged particles), they have
been seen at lower latitudes, including even in the
tropics! Even if they were apparent at our latitude of
34° N, we would probably miss the show, though,
because of light pollution from our city lights: You
would have to be out in the mountains or desert to see
them if they were down in our low latitudes.
v. Another side effect is that the ionosphere absorbs and
reflects radio waves. A layer of ions at the very bottom
of the ionosphere (largely oxygen) forms in the daytime and
can absorb a lot of radio waves. It disappears at night,
but dense ion layers higher up last longer into the night
and reflect radio waves, espeially short wave radio. This
allows radio stations to be picked up way beyond the
horizon from the broadcast tower, which is why you can pick
up radio broadcasts from Nevada, Nebraska, and Iowa at
night sometimes.
b. The exosphere is the second, outer layer of the thermosphere
Dick Hutchinson of Circle, Alaska, took this picture, and
you can see more of his work with aurorae there at:
http://www.ptialaska.net/~hutch/aurora.html.
a. These light displays are called the aurora borealis or
Northern Lights in the Northern Hemisphere and the
aurora australis or Southern Lights in the Southern
Hemisphere.
b. Normally, you have to be near the polar regions to see
them but, every once in a while, especially during the
peaks of the sunspot cycle (when the sun is emitting a
stronger solar wind of charged particles), they have
been seen at lower latitudes, including even in the
tropics! Even if they were apparent at our latitude of
34° N, we would probably miss the show, though,
because of light pollution from our city lights: You
would have to be out in the mountains or desert to see
them if they were down in our low latitudes.
v. Another side effect is that the ionosphere absorbs and
reflects radio waves. A layer of ions at the very bottom
of the ionosphere (largely oxygen) forms in the daytime and
can absorb a lot of radio waves. It disappears at night,
but dense ion layers higher up last longer into the night
and reflect radio waves, espeially short wave radio. This
allows radio stations to be picked up way beyond the
horizon from the broadcast tower, which is why you can pick
up radio broadcasts from Nevada, Nebraska, and Iowa at
night sometimes.
b. The exosphere is the second, outer layer of the thermosphere
 i. The exosphere lies beyond about 500-1,000 km and is
characterized by increasing hydrogen and helium content,
because the oxygen and nitrogen that dominate the lower
atmosphere have been dissociated into ions in the
ionosphere. We're not talking too many molecules and ions
way the heck up here. Density shades into the levels seen
in interplanetary space up around, oh, 10,000 km.
ii. Hydrogen and helium molecules can and do easily leave Earth
orbit for outer space from the top of the exosphere,
because at this altitude, when their trajectories are
changed by collision with another molecule to a generally
upward direction, they have less and less chance of
colliding with another one and being bounced back
earthward. So, there is a constant loss of H and He to
outer space from the upper exosphere, and Earth's gravity
is not strong enough to hold onto these lightest of all
gasses (unlike such big bruisers as Jupiter, Saturn,
Uranus, and Neptune, the gas giant planets in the outer
solar system, which retain hydrogen and helium atmospheres
with their tremendous size and gravity).
V. So, you've seen that the atmosphere has a distinct vertical structure,
which can be described in terms of a pressure gradient, as in the last
lecture, and in terms of its thermal structure and its physico-chemical
structure. I organized this particular lecture around the thermal
structure of the atmosphere but included discussion of the physico-
chemical layers within the thermal layers where they're found. So, the
ozone layer was discussed as part of the stratosphere, while the
ionosphere and exosphere were discussed under the thermosphere. There is
another distinction often made, too: We can differentiate the vertical
structure of the atmosphere by the balance of chemical mixing and
ionization:
A. The homosphere is that portion of the lower atmosphere with almost no
ionization and in which there are mixing mechanisms moving gases from
one part of the atmosphere to another, resulting in a fairly uniform
chemical composition: This is the lower 80-100 km or thereabouts
(troposphere through mesosphere).
1. The major variation you get in here is a greater concentration of
ozone in the stratosphere, but ozone is a non-ionized gas, even so.
2. Throughout the homosphere, then, nitrogen and oxygen gasses
dominate.
B. The heterosphere is the area in which you get varying mixes of gas
molecules and ions, and in which the nitrogen and oxygen that dominate
the lower atmosphere give way, through ionization, to hydrogen and
helium at the top of the atmosphere. That would be somewhere from 80
km to 10,000 km, where the hydrogen and helium gasses are so feebly
held to Earth that they are constantly being Lost in Space.
Come away from this lecture knowing the four different thermally-defined
layers of the atmosphere (troposphere, statosphere, mesosphere, and
thermosphere) and what sort of relation (direct or inverse) between
temperature and altitude is found in each of them. Know the three isothermic
belts (tropopause, stratopause, and mesopause) and what the dickens an
"isothermic belt" is.
Learn which physico-chemical feature is found in the four layers (weather in
the troposphere, ozone layer in the stratosphere, and the ionosphere and
exosphere in the thermosphere), and be able to explain the aurorae (Northern
Lights or aurora borealis and Southern Lights or aurora australis). Also,
know the difference between the homosphere and the heterosphere and which
thermally-defined layers belong to which.
i. The exosphere lies beyond about 500-1,000 km and is
characterized by increasing hydrogen and helium content,
because the oxygen and nitrogen that dominate the lower
atmosphere have been dissociated into ions in the
ionosphere. We're not talking too many molecules and ions
way the heck up here. Density shades into the levels seen
in interplanetary space up around, oh, 10,000 km.
ii. Hydrogen and helium molecules can and do easily leave Earth
orbit for outer space from the top of the exosphere,
because at this altitude, when their trajectories are
changed by collision with another molecule to a generally
upward direction, they have less and less chance of
colliding with another one and being bounced back
earthward. So, there is a constant loss of H and He to
outer space from the upper exosphere, and Earth's gravity
is not strong enough to hold onto these lightest of all
gasses (unlike such big bruisers as Jupiter, Saturn,
Uranus, and Neptune, the gas giant planets in the outer
solar system, which retain hydrogen and helium atmospheres
with their tremendous size and gravity).
V. So, you've seen that the atmosphere has a distinct vertical structure,
which can be described in terms of a pressure gradient, as in the last
lecture, and in terms of its thermal structure and its physico-chemical
structure. I organized this particular lecture around the thermal
structure of the atmosphere but included discussion of the physico-
chemical layers within the thermal layers where they're found. So, the
ozone layer was discussed as part of the stratosphere, while the
ionosphere and exosphere were discussed under the thermosphere. There is
another distinction often made, too: We can differentiate the vertical
structure of the atmosphere by the balance of chemical mixing and
ionization:
A. The homosphere is that portion of the lower atmosphere with almost no
ionization and in which there are mixing mechanisms moving gases from
one part of the atmosphere to another, resulting in a fairly uniform
chemical composition: This is the lower 80-100 km or thereabouts
(troposphere through mesosphere).
1. The major variation you get in here is a greater concentration of
ozone in the stratosphere, but ozone is a non-ionized gas, even so.
2. Throughout the homosphere, then, nitrogen and oxygen gasses
dominate.
B. The heterosphere is the area in which you get varying mixes of gas
molecules and ions, and in which the nitrogen and oxygen that dominate
the lower atmosphere give way, through ionization, to hydrogen and
helium at the top of the atmosphere. That would be somewhere from 80
km to 10,000 km, where the hydrogen and helium gasses are so feebly
held to Earth that they are constantly being Lost in Space.
Come away from this lecture knowing the four different thermally-defined
layers of the atmosphere (troposphere, statosphere, mesosphere, and
thermosphere) and what sort of relation (direct or inverse) between
temperature and altitude is found in each of them. Know the three isothermic
belts (tropopause, stratopause, and mesopause) and what the dickens an
"isothermic belt" is.
Learn which physico-chemical feature is found in the four layers (weather in
the troposphere, ozone layer in the stratosphere, and the ionosphere and
exosphere in the thermosphere), and be able to explain the aurorae (Northern
Lights or aurora borealis and Southern Lights or aurora australis). Also,
know the difference between the homosphere and the heterosphere and which
thermally-defined layers belong to which.